
DIABETES IN SKIN DISEASE
Art Huntley, M.D.
Rhett Drugge, M.D.
ABSTRACT
Diabetes mellitus is a common condition which frequently has skin
manifestations. The attachment of glucose to protein may result in a
profound effect on structure and function of that protein, and
account for clinical manifestations of the disease. It has been
suggested that increased crosslinking of collagen in diabetic
patients is responsible for the fact that their skin is generally
thicker than that of non-diabetics. Advanced glycosylation end
products are probably responsible for yellowing of skin and nails.
Increased viscosity of blood due to stiff red blood cell membranes
results in engorgement of the post-capillary venules in the papillary
dermis, detected as erythema of the face, or periungual erythema. It
is suggested that these skin changes may eventually be used as a
reflection of the patient's current as well as past metabolic status.

Introduction
Skin manifestations in diabetes mellitus are common and expressed
in numerous forms. If one considers metabolic effects on
microcirculation and changes in skin collagen, prevalence approaches
100 percent. Findings range from the presenting manifestations of the
disease to signs of long term involvement, from the mundane to
indications of serious, even life-threatening problems. For all of
these, recognition is the key to treatment and/or prevention. This
review of cutaneous manifestations of diabetes groups findings
according to presumed pathophysiology. Since the pathophysiology is
not always known, some less common findings are discussed separately
at the end.
Biochemical Considerations
In 1968 Rahbar published the observation that patients with
diabetes mellitus have oddly behaving hemoglobin.(1)
This was subsequently demonstrated to be due to non-enzymatic
condensation of glucose with hemoglobin to form stable covalent
adducts. Non-enzymatic glycosylation occurs with many proteins
including hemoglobin, an attachment that results in changes in the
physical and chemical properties.
Glucose in solution exists as a stable pyranose ring in
equilibrium with the open chain aldehyde form. The reaction of
monosaccharides with proteins consists of the covalent linkage of the
double-bonded oxygen of the aldehyde function with an NH2 group,
either on the alpha-amino group of the N-terminal amino acid or on
the epsilon-amino group of lysine. This condensation results in the
formation of a Schiff base or aldimine, and is a reversible reaction.
However, following the formation of the Schiff base, there is an
internal reconfiguration of the molecule, the so called Amadori
rearrangement, resulting in formation of a ketoamine which tends to
not revert back to the Schiff base. The rate of reaction of various
carbohydrates with protein correlates with the extent to which the
sugar exists in the open ring (aldehyde) form.
Following the condensation and reconfiguration, the Amadori
products undergo a series of further reactions with amino groups on
other proteins to form glucose-derived intermolecular crosslinks.
(2) These collagen modifications result in a color
change which has been demonstrated by spectrophotometric measurement
to correlate with diabetic complications. (3) One of
these advanced glycosylation products, a yellow compound, 2-(2-
furoyl)-4(5)-(2-furanyl)-1H-imidazole, has been identified.
(4) Quantitation of another advanced glycosylation
end product in the skin, the amino acid pentosidine, has also been
demonstrated to correlate with a cumulative score of diabetic
complications. (5)
The process of non-enzymatic glycosylation occurs to a minor
extent at normal blood sugar concentrations. This gradual
glycosylation of proteins may be responsible for some of the skin
changes associated with aging, and this process is apparently
accelerated in persons with elevated blood sugars. Most proteins
evaluated seem to be involved by this reaction which results in
changes in the physical and chemical properties. Glucosylation of the
red cell membrane is apparently responsible for the stiffness of
diabetic erythrocytes. (6) Glucosylation of collagen
results in increased stiffness and resistance to enzymatic
degradation, mechanical changes of collagen which are also
characteristic for aging.
Protein glycosylation with changes in tertiary structure and
solubility of proteins could conceivably be responsible for many of
the complications of this disease.
Genetics of Diabetes: Several exemplary
syndromes
Wolfram's or DIDMOAD syndrome (OMIM
) (diabetes insipidus, diabetes mellitus, optic atrophy, and
nerve deafness). (autosomal recessive and associated with non-immune
complete and selective beta cell destruction and severe and
progressive neuronal loss. Onset of diabetes is often in infancy.
This syndrome appears to be responsive to thiamine. There are no
characteristic skin findings reported. Wolfram syndrome is a rare
inherited disorder that leads to an array of symptoms, including
diabetes mellitus and blindness. More important, the syndrome's
victims usually suffer from severe nervous system abnormalities that
can be accompanied by behavior problems, psychiatric hospitalizations
and--in 25 percent of cases--suicide attempts. Linkage analysis
indicates that the likely location of the Wolfram gene is the short
arm of chromosome 4. Between one in 50,000 to 100,000 people in this
country inherit Wolfram syndrome. Although the syndrome itself is
rare, experts estimate that about 1 in 100--or as many as 2.5 million
Americans--possess a single copy of the mutated gene. Because these
individuals, as well as close relatives of people with Wolfram
syndrome, experience higher-than-normal rates of psychiatric illness.

Maturity onset Diabetes of Youth (MODY) syndrome (OMIM
). Variants of this syndrome may be very common in American
blacks and individuals from India. In some families inheritance is
autosomal dominant. Chlorpropamide-alcohol flushing may be a marker
for this form. 
Hemochromatosis (OMIM
). This is associated with the development of diabetes, and the
autosomal recessive gene causing hemochromatosis is located within
the major histocompatibility complex and is associated with HLA
antigens A3 and B14. Patients with this syndrome often have insulin
resistance in association with other manifestations of iron overload
(bronzing of the skin, hepatomegaly, and cirrhosis). Affected
asymptomatic individuals can now be identified even prior to
increased serum ferritin, since the one in four siblings HLA
identical to a hemochromatotic sibling are almost always homozygous
for the involved gene. The gene causing hemochromatosis is very
common in the general population (almost 10%); thus, approximately
2.5% of offspring of a patient with hemochromatosis will develop
hemochromatosis. The sequelae of iron overload are preventable with
simple phlebotomy, and therefore it is important to screen all first
degree relatives of patients with hemochromatosis for abnormal iron
metabolism (e.g., transferrin saturation, ferritin levels). 
In secondary forms of iron overload including transfusional
hemosiderosis, alcoholic cirrhosis, thalassemia, sideroblastic
anemia, and porphyria cutanea tarda (OMIM
), iron accumulates in the reticuloendothelial system initially,
but with increasing amounts of total body iron, excessive iron
deposits eventually accumulate in parenchymal cells throughout the
body producing a picture indistinguishable from hereditary
hemochromatosis. Subnormal activity of hepatic uroporphyrinogen
decarboxylase is responsible for the derangement of porphyrin
biosynthesis in both sporadic and familial porphyria cutanea tarda,
but the enzymatic defect is not clinically expressed in the absence
of hepatic siderosis Pedigree studies support the hypothesis that
HLA-linked hemochromatosis alleles are far more common inpatients
with sporadic porphyria cutanea tarda than in individuals in the
general population and may be responsible for the hepatic siderosis
associated with most cases of sporadic porphyria cutanea tarda.

In a study of the skin manifestations of idiopathic
hemochromatosis in 100 cases, there was a high frequency of
ichthyosis-like states and koilonychia. In 50 cases with treated and
non-treated groups, histological siderosis and clinical skin
pigmentation were found to decrease post-phlebotomy whereas melanosis
did not. Siderosis of eccrine sweat glands provided a probable
diagnosis of the disease. Necrobiosis lipoidica and a black
keratinous cyst have also been reported. 
Porphyria cutanea tarda, clinical (1 , 2 ) .
The latter is more commonly seen, producing photosensitivity in the
exposed areas, e.g., bullae on the dorsa of the hands, showing pink
urine with the Wood's lamp examination. This condition usually is
seen in liver damage from barbiturates, contraceptive pills,
estrogens, alcohol or diabetes. The treatment is
phlebotomy.
|

|
These photos illustrate the facial telangiectasis
associated with increased in systemic estrogens.
|
|

|
Blisters of porphyria cutanea tarda (PCT)
in the most common region, the dorsal hands. PCT also
features facial hypertrichosis as a prominent sign of
disease.
|
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophies an
autosomal recessive disease characterized by a variable combination
of (1) failure of the parathyroid glands, adrenal cortex, gonads,
pancreatic beta cells, gastric parietal cells, and thyroid gland with
associated myxedema, and hepatitis; (2) chronic
mucocutaneous candidiasis; and (3) dystrophy of dental enamel, nail
pitting, alopecia areata, vitiligo and ocular keratopathy. This
disease has many names, one being autoimmune polyglandular disease
Type I. 
|
The photographs at the right
illustrate several of the more common autoimmune conditions
seen in autoimmune polyglandular disease Type
I
|

|

|

|

|
In a recent study of 68 patients form 54 families, the clinical
manifestations varied greatly and included from one to eight disease
components, 63 percent of the patients having three to five of them.
The initial manifestation was oral candidiasis in 41 patient (60%).
The earliest endocrine component appeared at 19 months to 35 years of
age. Eight patients (12%) had insulin-dependent diabetes mellitus,
with the age at onset ranging from 4.1 to 37 years. Nine patients had
vitamin B12 deficiencies that began at the ages of 6.1 to 47. Two
female patients had non-goitrous hypothyroidism; no other patient had
any thyroid autoimmune disease. 
All 68 patients had oral candidiasis at least periodically; this
condition first appeared at ages ranging from one month to 21 years.
In six patients the candidiasis was very mild and disappeared
spontaneously for as long as several years, but it always recurred.
Other patients had chronic hypertrophic lesions, atrophic lesions, or
both. Ungual candidiasis was present in 48 patients, and dermal
candidiasis in six. The lesions were usually located on the hands and
face. Four patients had esophagitis that was diagnosed by endoscopy,
with stricture in one. Eleven other patients reported periods of
retrosternal pain that resolved within days after the initiation of
oral antifungal therapy. Ketoconazole 200 mg PO daily was effective
in a double-blind trial; all six ketoconazole-treated patients showed
clear clinical and mycological improvement, of oral and nail
involvement. Death due to metastatic squamous cell carcinoma of the
oral mucosa has been reported. 
77% of patients had hypoplasia of the dental enamel that had begun
at birth or during the first seven years. 2% had pitted dystrophy of
the nails. The pits were 0.5 to 1 mm in diameter and affected several
nails; The surface of the nail was otherwise smooth. 
Acanthosis nigricans
|

|
Acanthosis nigricans is characterized by symmetric,
velvety to verrucous, hyperkeratotic and hyperpigmented
plaques that have a predilection for the axillae, the nape,
and other flexural areas.The degree of cutaneous involvement
varies from subtle hyperpigmentation and papillary
thickening, affecting few areas, to a deeply pigmenting and
verrucous process that involves the entire integument,
including mucous membranes, palms, and soles. Light
microscopy distinguishes the disorder by the presence of
hyperkeratosis, papillomatosis, area of acanthosis that may
alternate with areas of atrophy, and increased amounts of
melanin in the basal epidermis.
|
|

|
The relationship between insulin resistance and acanthosis
nigricans is especially clear in the context of normal and disordered
insulin metabolism. Insulin facilitates the uptake of glucose into
most cells, regulates fat and protein metabolism, and promotes DNA
synthesis and cell growth. Many of these functions are thought to be
modulated by the binding of insulin to the insulin receptor, a
membrane glycoprotein with intrinsic tyrosine-kinase activity. In
addition, insulin can bind to the receptors composed of insulin-like
growth factors. Insulin-like growth factors are peptides with
structures homologous to that of insulin, and like insulin, these
peptides have growth-promoting effects. In recent studies it has been
suggested that the growth-promoting effects of insulin at low
concentrations are medicated by "classic" receptors, whereas effects
at high concentrations are mediated by insulin-like growth factor
receptors.
High plasma levels of insulin are thought to contribute to the
development of acanthosis nigricans. Evidence for this hypothesis
include the following observations: classic insulin receptors and
insulin-like growth factor receptors have been identified in cultured
human fibroblasts and keratinocytes. Localized acanthosis nigricans
has been noted at sites of subcutaneous administration of insulin for
the treatment of diabetes mellitus. Finally, many of the conditions
associated with acanthosis nigricans have been linked to the presence
of some form of insulin  resistance.
resistance.
Non-familial
congenital syndromes associated with acanthosis nigricans
|
Alström syndrome
|
Ataxia-telangiectasia
|
Bloom syndrome
|
|
Capozucco syndrome
|
Crouzon's disease (craniofacial dysostosis)
|
Lawrence-Seip syndrome (total lipodystrophy)
|
|
Leprechaunism (70)
|
Prader-Willi syndrome
|
Rabson's syndrome
|
|
Rud's syndrome
|
Syndrome of acral hypertrophy and muscle cramps
|
Polycystic ovary disease (Stein-Leventhal syndrome)
|
Endocrine disorders associated with insulin resistance and acanthosis
nigricans:
|
Acromegally
|
 
|
|
Others Not Represented Here
|
|
Ovarian hyperthecosis
|
|
Cushing's disease
Hypothyroidism
|
Obesity Pinealoma or pineal hyperplasia
(Rabson-mendenhall) (
OMIM)
|
|
|
Table I. Drugs associated with
acanthosis nigricans:
|
Nicotinic acid
|
Diethylstilbestrol
|
Glucocorticoids
|
CUTANEOUS INFECTIONS IN DIABETES
MELLITUS
In the pre-insulin era the prevalence of common pyodermas such as
furunculosis, carbunculosis, and erysipelas was much higher for
diabetics than for their non-diabetic counterparts. (7)
Today, these infections do not seem to result in much morbidity and
diabetics do not even appear to have an increase in the prevalence of
skin infection. (8) However, there are several
infections which characteristically occur in persons with diabetes
mellitus, and some threaten life and limb.
Candida Infections
Yeast infections are common in diabetic patients. Involvement of
the glans penis and of the vulva appear common in type II diabetes.
Vaginal candidiasis is almost universal among women with long term
diabetes, and yeast infections may even be the presenting
manifestation of diabetes.(9)
Vulvo-vaginal candida infection is an especially common problem
for the diabetic woman. (10) It is a common cause
of pruritus vulvae during glycosuria. Presenting signs include vulvar
erythema which may be accompanied by fissuring with or without
satellite pustules. Vaginitis is usually accompanied by a white
discharge. Traditional treatment involves normalizing blood sugar,
treating both the vagina and vulva with topical medication. Since
these patients often have a reservoir of Candida in the colon, oral
nystatin may also be administered. Another option for vaginal
candidiasis is oral administration of one dose 150 of mg of
fluconazole.
Angular stomatitis due to Candida is a classic complication in
diabetic children and an occasional complication in diabetic adults.
Increased concentrations of salivary glucose reportedly accounts for
its occurrence, (11) but not for the predilection
for younger patients. Clinically it is appreciated as white, curd-
like material which adheres to erythematous, fissured areas at the
angle of the mouth or as white patches on the buccal mucosa and
palate. Diagnosis is readily confirmed by examination of a potassium
hydroxide preparation. Success in treatment may depend on
normalization of blood sugar and the supplemental use of anticandidal
lozenges.
The prevalence of Candida infection of the hands and feet does not
appear to be significantly different for the diabetic population as
compared to controls. (12) When it does occur, it
usually has one of three presentations. Candida paronychia usually
involves the hands but it may occur on the feet. It often begins at
the lateral nail folds as erythema, swelling, and separation of the
fold from the lateral margin of the nail. Further infection may
result in involvement of the proximal nail fold and separation of the
cuticle from the nail. Moisture trapped in the resultant space favors
further growth of the yeast and repeated episodes of inflammation. At
times there may be a purulent discharge from involved nail fold, a
clinical finding suggesting bacterial paronychia. But the diagnosis
of yeast infection can usually be established by performing a KOH
preparation on extruded serous material from this space.
Candida infection of the web spaces usually involves the 3-4 web
space of the hands or the 4-5 web-space of the toes. This area has a
tendency to retain moisture due to occlusion from apposing surfaces
of skin. Presumably the increased sugar content of the skin
encourages the establishment of this infection. The clinical
appearance is a white patch of skin, often with central peeling. Toe
web space involvement is often mistaken for a dermatophyte infection,
but the diagnosis can be confirmed on potassium hydroxide
preparation.
The third presentation of Candida infection of the extremities is
involvement in the toe nail plates. Although dystrophic toe nails are
often assumed to be the result of dermatophyte infection, nail plate
cultures demonstrate the pathogen to be Candida species about five
percent of the time. One needs to be careful about making the
diagnosis of primary Candida infection, however, because cultures may
only reflect contaminants or secondary involvement.Clinically, nail
plate infections with either dermatophyte or Candida sp. present with
distal yellowing or whitening and thickening of the toe nail. Living
tissue does not appear to be involved. If there is a special risk to
the diabetic host to have this nail plate infection, it has not been
demonstrated.
|

|
Chronic Candidiasis of the fingernails
|
Gangrene in
Diabetes
|

|
Hyperglycemia can allow usually nonpathogenic
organisms to establish an infection in traumatized skin,
occasionally resulting in gangrene and loss of limb.
|
Phycomycetes
Infections
Diabetic patients with leg ulcers, or non-healing surgical wounds,
especially those of the lower extremities, may have a complicating
Phycomycetes infection. Such an infection should be suspected when
lower extremity ulcers or post-traumatic lesions are not responding
to therapy. Diagnosis can be confirmed by culture and by histologic
demonstration of fungal elements invading vascular channels.
Patients with uncontrolled diabetes with ketosis may be
predisposed to deep mycotic infections such as the rare but serious
forms of mucormycosis. The characteristic presentation is black
crusting or pus on the turbinates, septum, or palate. Without
treatment the infection may extend to the maxillary and ethmoid
sinuses, the palate, and the orbit. Cerebral involvement occurs in
about two thirds of these patients. (13) Treatment
consists of correction of acid-base imbalance, aggressive debridement
of necrotic tissue, and intravenous amphotericin.
Pseudomonas Infections
Malignant external otitis, an uncommon, but serious, infection of
the external ear canal by Pseudomonas, characteristically presents as
and severe external ear canal pain and purulent discharge in an
elderly diabetic patient. (14,15)
The infection is thought to begin as a cellulitis of the ear canal,
but natural cleavage planes allow progression through the osseous
cartilaginous junction. With further extension the cranial nerves may
be involved, especially the facial nerve. About half the affected
individuals die of this infection. Treatment of choice consists of
surgical debridement and administration of anti-pseudomonas
antibiotics. 
Much more common than malignant external otitis, however, is
Pseudomonas infection of the toe web spaces or colonization under the
toenails. Often, persons who have onychomycosis develop a lifting of
the nail plate form the bed (onycholysis). The resulting space
between plate and bed may become colonized with Pseudomonas resulting
in a green discoloration of this area. 
Pseudomonas may cause web space infection on the feet similar to
that due to dermatophytosis, but this assumption may be incorrect.
The differential diagnosis includes candidiasis, infection due to
Pseudomonas, but a Wood's lamp examination often yields a green
fluorescence. Soaks with dilute vinegar may eradicate superficial
infection, with more advanced cellulitis, oral Ciprofloxacin appears
to be the treatment of choice. 
Dermatophytosis
Although dermatophyte infections are probably not more common in
the diabetic population, (16) they are of special
concern. Toe web space infections may lead to inflammation and
fissuring that can serve as a portal of entry for bacterial infection
in a compromised diabetic foot. The oxygen demand of the subsequent
inflammation may exceed the ability of the diabetic microcirculation,
leading to gangrene. It is for that reason that tinea pedis should be
aggressively managed in patients with neurovascular
compromise.
Involvement of the toe-nails by dermatophytes (onychomycosis) is
common among elderly diabetics as it is in the population at large.
The infection itself is of little consequence, but the nail dystrophy
which results may make proper nail care more difficult for the
patient. Recently the FDA approved both
Itraconazole treatment (200 mg/day for one week a month for 4 months)
and Terbinafine (250 mg/day for 3 months.)
DERMAL MANIFESTATIONS OF DIABETES MELLITUS
Diabetic Thick
Skin
Persons with diabetes tend to have thicker skin than their
non-diabetic counterparts. There are three aspects to this
observation. First, diabetics in general have a clinically inapparent
but measurable increase in skin thickness unassociated with symptoms
and goes unnoticed by patients and physicians. Second is a clinically
apparent thickening of skin involving the fingers and hands ranging
from pebbled skin to scleroderma-like skin changes. And third is an
infrequent syndrome of diabetic scleredema in which the patient
develops markedly thickened dermis on the upper back region.
The presence of diabetes mellitus is generally associated with
measurably thickened skin. Using pulsed ultrasound, it can be
demonstrated that diabetics have thicker forearm skin than their age
and sex-matched nondiabetic counterparts.(17)
Contrary to the pattern in non-diabetics, skin thickness may increase
with age (apparently associated with increased duration of diabetes.)
Most studies have used the upper extremity skin in evaluating skin
thickness, and it may not be a valid conclusion that diabetic skin is
thickened at other sites. We have also demonstrated an increased skin
thickness on the dorsum of the feet, but not the back, suggesting
that increased skin thickness is not necessarily universal in
diabetes.(18) It appears to be a safe observation
that patients with diabetes mellitus have thicker skin on their
extremities.
Hand Skin Thickening
Thickening of skin of the hand is a common occurrence, with a
range of manifestation from simple pebbling of the knuckles to the
diabetic hand syndrome. The diabetic hand syndrome consists of
thickened skin over the dorsum of the digits and limited joint
mobility, especially of the interphalangeal joints.(19)
The earliest description of this phenomenon was apparently the
observation that insulin- dependent diabetes was occasionally
complicated by painful stiff hands.(20)
Subsequently, Rosenbloom and Frais described three adolescent
patients with the syndrome of long-standing diabetes mellitus,
restricted joint mobility, thick tight waxy skin, growth impairment,
and maturational delay.(21) Rosenbloom et al later
reported in a study of 309 mostly juvenile diabetics that 30 percent
had joint limitation and one third of these had thick tight, waxy
skin that the examiner could not tent, mostly involving the dorsum of
the hands.(22) This work has been confirmed by
other authors and these observations have been extended to patients
with type II diabetes mellitus.(23)
More common is simple thickening of the skin on the dorsum of the
hands. At least thirty percent of diabetic patients have hand skin
thickening, and some have demonstrable involvement of the dorsum of
the feet. Clinical clues which suggest such a thickening include
difficulty in tenting the skin, pebbled or rough skin on the knuckles
or periungual region,(24) and decreased skin
wrinkling following immersion in water.(25)
|

|
Knuckle Pebbles. Thickening of the skin on the dorsum
of the hand due to many causes, diabetes among them, is
often manifest by a pebbling of the skin on the knuckle
area. This patient with insulin dependent diabetes mellitus
also has palpably thick skin when tenting is
attempted.
|
. What is the significance of thick skin on the hands and feet in
diabetes mellitus? The literature suggests that digital sclerosis
(very thick skin) is a marker for retinal microvascular disease. But
there is a spectrum of thickening of the skin which ranges from that
which is only detectable by ultrasound to the more obvious. For less
than digital sclerosis, the significance of thick skin in diabetes is
uncertain at this time.
Scleredema of Diabetes
|

|
Scleredema of Diabetes. This patient with Type II
diabetes gave an incidental complaint of limitation of
motion of his upper extremities. To examination, this
appeared to be due to the shield-like involvement of the
upper back, neck, and shoulders with marked dermal
thickening. The examiner is demonstrating the inability to
tent the skin on the dorsum of the neck.
|
Scleredema adultorum of diabetes is a syndrome characterized by a
marked increase in dermal thickness on the posterior back and upper
neck in middle aged, overweight, poorly controlled type II diabetic
subjects. It is not recognized a being related to digital sclerosis,
and we found no correlation by ultrasound measurements of back skin
and hand skin thickness. It has a reported prevalence of 2.5 percent
in patients with type II diabetes.(26)
Histologically one finds a thickened dermis with large collagen
bundles that are separated by wide, clear spaces. There may be
increased numbers of mast cells. (27) There are
reports of increased, normal, and decreased glycosaminoglycans in
affected dermis.(28)
Are there any known treatments for the thick skin syndromes? There
is one study which suggests that tight control of blood sugar helps.
Lieberman et al reported that four diabetic patients with thick skin
had a decrease of skin thickness following pump administration of
insulin and achievement of tighter control.(29) In
that study skin thickness was measured ultrasonically on six body
areas and the sums of pre- and post- treatment determinations were
compared. However, they did not report thickness measurements for any
one area. There is no known treatment for diabetic
scleredema.
Yellow Skin
Diabetic skin often has a yellow hue. Traditionally considered to
be carotenemia, recent evaluations indicate that serum carotene
levels are not elevated as they had been years ago when the standard
diabetic diet involved heavy consumption of vegetables.(30)
One possible cause of yellow skin might be glycosylation end
products. It is known that proteins which have a long turnover time,
such as dermal collagen, undergo glycosylation and become yellow. One
of the advanced glycosylation products which has been identified,
2-(2-furoyl)-4(5)-(2-furanyl)-1H-imidazole, has a distinctly yellow
hue (see the earlier section on Biochemical Considerations).
Yellow skin is a common finding among patients with diabetes,
probably best appreciated on the palms and soles because of sparse
competition with melanocytic pigment in these areas. There is
currently no significance associated with this finding other than
that of a time proven observation.
VASCULAR MANIFESTATIONS OF DIABETES MELLITUS
Macroangiopathy
Diabetics have a higher incidence and prevalence of large vessel
disease,(31) and develop myocardial infarctions and
strokes at a much younger age than their non-diabetic counterparts.
Large vessel disease (atherosclerosis) may also be present in the
lower extremities and result in skin atrophy, hair loss, coldness of
the toes, nail dystrophy, pallor upon elevation, and mottling on
dependence. (32)
Microangiopathy
Microangiopathy is one of the major complications of diabetes
mellitus. The small blood vessel changes affecting the retinal and
renal vasculature are responsible for blindness and kidney failure
Microvascular pathology has also been assumed to play a role in
diabetic neuropathy, and in the so-called diabetic foot.
Microangiopathy is clinically detected by an eye ground examination
which demonstrates the presence of microaneurysms. More severe
involvement may demonstrate hemorrhages, exudates, and even some
devascularized areas as well.
|
 
|
Kyrle's Disease: an uncommon finding in patient
receiving renal dialysis.
The skin is extruding collagen in this disorder which is
much more prevalent in diabetes.
|
The histology of affected diabetic tissue reveals a PAS positive,
thickened capillary basement membrane. Electron microscopy of
skeletal muscle capillaries reveals reduplication of the basal
lamina. The skin has not been thought to be a good sample source in
evaluation of patients microangiopathy because small blood vessels of
the dermis develop less basal lamina thickening than is found in
skeletal muscle (which is also easily accessed using a needle
biopsy).
The structural changes which occur in the microcirculation do not
seem to account for all the full extent of the disease, leading to
the concept of functional microangiopathy. Some patients with severe
microcirculatory problems such as gangrene of the foot have normal
capillaries on skin or skeletal muscle biopsy. Sluggish
microcirculation resulting in micro-venular dilatation is considered
"functional" in that it may be reversed with improved control of
diabetes. The clinical manifestations associated with this include
retinal venous dilatation, red face, and periungual telangiectasia,
all of which may be very early manifestations of the disease and
which may improve with control of diabetes.
Functional microangiopathy may result from nonenzymatic
glycosylation which affects many blood components including
hemoglobin, red blood cell membrane, fibronectin, fibrinogen, and
platelets. Glucosylation of the red blood cell has been shown to
inhibit the cell pliability and to decrease the ability of this cell
to pass through pores smaller than 7 microns. The lumen of some
capillaries may be as narrow as 3 microns and ordinarily red blood
cells will elongate into a more sausage like configuration to
traverse this loop. Stiffened membranes will certainly inhibit or
limit this passage.
In addition to stiffened red blood cells, diabetics also have
increased plasma concentration of fibrinogen and capillary leakage
leading to loss of albumen and water. There is an increased tendency
for diabetic platelets to aggregate. The end result is increased
whole blood or plasma viscosity and sluggish
microcirculation.
In summary, it appears that microangiopathy can be attributed to
both structural and functional abnormalities in these vessels. The
following discussion will review some of the cutaneous manifestations
which may be linked to this microangiopathy.
Diabetic
Dermopathy
|

|

|
Diabetic Dermopathy. The presence of many
hyperpigmented atrophic macules on the shins is said to be a
relatively common finding in patients with diabetes.
Antecedent trauma may or may not be recalled by the
patient.
|
Atrophic hyperpigmented macules on the shins, so-called diabetic
dermopathy, has been termed the most common cutaneous finding in
diabetes.(33) It is usually noted as irregularly
round or oval, circumscribed, shallow lesions vary in number from few
to many, which are usually bilateral but not symmetrically
distributed. They are asymptomatic and often overlooked.
The genesis of these lesions is unclear. Some authors describe a
preceding, distinct, red papular eruption which is independent of
trauma to the skin.(34) However, Lithner has been
able to duplicate these lesions by local thermal trauma.(35)
We observe that many patients who develop these depressed
hyperpigmented lesions relate antecedent trauma or mild pyoderma such
as folliculitis. "Diabetic dermopathy" probably represents
post-traumatic atrophy and post-inflammatory hyperpigmentation in
poorly vascularized skin.
Do these lesions represent the cutaneous manifestation of
structural microangiopathy? Histologic characteristics of acute
lesions are edema of the epidermis and papillary dermis, extravasated
erythrocytes and a mild lymphohistiocytic infiltrate.(36)
Older lesions have thick-walled capillaries in the upper dermis,
occasional extravasated erythrocytes and a positive Perl stain for
iron. However, one electron microscopic study demonstrated only in
some patients the presence of thickened basal lamina.(37)
Based on the available studies, there appear to be structural
components and some suggestion of a functional factors as
well.
The significance and prevalence of diabetic dermopathy depends on
the operational definition of this entity. Defined as one or more
spots, the original description reported their presence in 55% of 293
diabetics (65% of males and 29% of females).(38)
But with this definition, it has also been shown to occur in 20% of
control patients with normal glucose tolerance tests.(39)
Thus, defining diabetic dermopathy as one or more spots results in
high sensitivity but low specificity for diabetes. However, in a
study which defined dermopathy as the presence of four or more
lesions, they were absent in non-diabetics and present in about 14%
of diabetics (24% of men and 3% of women).(40) The
multilesional definition also found a high correlation with
retinovascular disease.
Pigmented
Purpura
|

|
Pigmented Purpura. Salt and Pepper type of yellow-tan
hyperpigmentation of the shins in the absence of atrophy is
characteristic for pigmented purpura, a common finding
especially in elderly diabetics. Patients need not be
diabetic to demonstrate this finding. This finding is often
seen in conjunction with diabetic dermopathy so there are
some areas of focal atrophy and wide areas of non-atrophic
pigmentation.
|
Pigmented purpuric dermatosis is a condition involving the skin on
the lower extremities resulting from red blood cell extravasation
from the superficial vascular plexus. It is characterized by multiple
tan to reddish small macules (so-called cayenne pepper spots) which
coalesce into tan to orange patches. It often extends down to involve
the ankles and the dorsum of the feet. It was described as a
manifestation in older diabetic patients, about half of whom had
diabetic dermopathy.(41) In most of these patients,
cardiac decompensation with edema of the legs was determined to be a
precipitating factor for the purpura. Except for the frequent
association with diabetic dermopathy, this condition appears
clinically consistent with Schambergs disease. Again, with little
insight into the pathophysiology, this condition appears to be a
marker of structural microangiopathy.
Red Skin and Rubeosis Facei
The prototype functional microangiopathy is facial involvement,
the so-called rubeosis facei. The intensity of red coloration which
can be appreciated in one's 'complexion' is a function of the degree
of engorgement of the superficial venous plexus. Hyperglycemia
predisposes to sluggish microcirculation and affected individuals
develop a functional microangiopathy which is clinically evident by
venous dilatation.(42) This venous dilatation can
be demonstrated in the eye grounds and skin. It may be evident in
newly diagnosed diabetics and, more importantly, the vascular
engorgement may return to normal when the blood sugar is controlled.
In a prospective study of 150 medical hospital admissions, comparing
facial redness (none, slightly red, or markedly red) with diabetic
parameters (persistent fasting hyperglycemia or a diabetic glucose
tolerance curve), of sixty one patients with diabetes, thirty-six
(59%) had markedly red faces.(43) Because of normal
variation in complexion, this sign is difficult to use as a marker of
functional microangiopathy.
Periungual
Telangiectasia
|

|
Periungual Telangiectasia. The nail fold is an
excellent site for viewing functional and structural changes
in the microvascular of the skin. This patient illustrates
microvascular engorgement and tortuosity involving the
proximal nail fold.
|
One may directly examine the skin to survey the superficial
microcirculation. Any area of skin may be examined, but because
nailfold capillary loops are in a horizontal axis relative to the
skin surface, this area offers an excellent view of the entire
microvascular loop. In order to see past the stratum corneum, it is
helpful to first apply mineral oil to the skin surface and wait a few
minutes until this layer becomes translucent. One may use a low power
microscope or simply an ophthalmoscope (+40 lens for 10X
magnification). In general, the microcirculation of less pigmented
individuals is often easier to visualize.
One study found venous capillary dilatation in the nail folds of
49% of seventy-five diabetic patients compared to 10% of sixty-five
controls.(44) It is important to note that
connective tissue diseases may also result in periungual vessel
changes, but that these changes are morphologically different. In
diabetes one sees isolated homogeneous engorgement of the venular
limbs. In connective tissue diseases, the patterns seen are
megacapillaries or irregularly enlarged loops.(45)

Venous dilatation of the periungual microcirculation appears to be
an excellent indicator of functional microangiopathy. The structural
changes of this area are probably represented by venous tortuosity.
Thus a newly diagnosed patient is likely do have simple capillary
loops with a dilated venous portion. A long term diabetic patient who
had poor control for a number of years, but who now has excellent
control, may exhibit venous tortuosity without dilatation. More
extensive microangiopathy can be heralded by small hemorrhages and by
drop-out of areas of the microcirculation.
Erysipelas-Like Erythema
Another reported phenomenon of microcirculatory compromise in
diabetic patients is the development of well demarcated erythema on
the lower leg or dorsum of the foot that correlates with radiological
evidence of underlying bone destruction, and incipient gangrene.
(46,47) The condition was at
first mistaken for erysipelas (hence the name erysipelas-like
erythema), but there was no associated pyrexia, elevated erythrocyte
sedimentation rate, or leukocytosis. This erythema would seem to be
functional microangiopathy localized to an area of macrocirculation
compromise.
OTHER SKIN MARKERS OF DIABETES MELLITUS
Yellow Nails
|

|
Yellow nails of diabetes. For comparison, the
examiners thumbnail is photographed alongside the yellow
nail of a person with diabetes. Another illustration of the
nail of the hallux of a person with diabetes is also
given.
|
As pointed out by Lithner, diabetics tend to have yellow
nails.(47) He noted this phenomenon in half of 36
diabetics and in none of nine controls. Our patients have a similar
prevalence of yellow nails, except we also see it occasionally in
elderly controls, and in some patients with onychomycosis. Although
this phenomenon may occur on all the nails, it is most often evident
on the distal aspect of the nail of the hallux.
What might account for this coloration? Clinically the yellow
color is not usually the result of underlying dermatophytosis.
Similar to the yellow color observed in diabetic skin, yellowing of
the nails probably represents glycosylation end products. Whereas
keratin of the epidermis is only present for one month before being
shed, that of the nail plate may be present for greater than a year.
The protein- glucose reaction presumably continues to evolve in the
aging nail resulting in the most yellow pigment at the distal aspect
of the slowest growing nail. The presence of the yellow glycosylation
end products in the nail plate has not been confirmed to date, but
one study of fingernails has demonstrated that diabetics have high
levels of fructose-lysine, another marker of nonenzymatic
glycosylation.(48)
Clinically one appreciates yellow nails of diabetes best on
examination of the toe-nails. Most diabetic patients have some aspect
of this yellowing. Minimal involvement consists of distal yellow or
yellow-brown discoloration of the hallux nail plate. Marked
involvement consists of canary yellow discoloration of all toe- and
finger-nails. It is not a specific finding in diabetes mellitus since
it can be occasionally observed with normal aging. Like the yellow
hue appreciated generally in the skin of persons with diabetes, the
significance of this observation is undetermined. The obvious
question is whether or not yellow nails and yellow skin can be used
as an quantifiable indicator of the degree of nonenzymatic
glycosylation for other tissues of the body.
Diabetic Bullae

|

|

|

|
Diabetic bullae. Adult onset
diabetic patient may have spontaneous onset of
multiple bullae on his lower extremities, one of which is
illustrated here. These lesions were not secondary to trauma
or infection and they healed without special intervention.

|
Another curious phenomenon in diabetes mellitus is the spontaneous
appearance of blisters on the extremities (usually confined to hands
or feet). These lesions are not the result of trauma or infection.
They tend to heal without treatment.
On the basis of cleavage level, there appears to be three types of
these blisters. The most common type is spontaneous and nonscarring.
They present as clear, sterile blisters on the tips of the toes or
fingers and less frequently on the dorsal and lateral surfaces of the
feet, legs, hands, and forearms. Spontaneous healing occurs within 2
to 5 weeks.(49) These patients were reported to
have good circulation to the affected extremity and tended to have
diabetic peripheral neuropathy. In those patients in whom
histopathology has been performed, there is an intraepidermal
cleavage without acantholysis.(50,51)
The second type of diabetic bullae involves lesions that may be
hemorrhagic and heal with scarring and atrophy.(52,53
The reported cleavage plane is below the dermoepidermal
junction.
A third type described in a case report consists of multiple
tender nonscarring blisters on sun-exposed and deeply tanned skin, on
the feet, legs, and arms. Immunofluorescence and porphyrin studies
were negative. Electron microscopy placed the cleavage plane at the
lamina lucida. (54)
Necrobiosis Lipoidica
The incidence is 0.3% in diabetics, and it is rare in
non-diabetics. The condition is most common between the second and
fifth decades of life, but it may be seen at any age. 80% of patients
with NLD are women. NLD occurs almost exclusively in whites.

between 60 and 65% with NLD will have overt diabetes at the time
of the diagnosis. Of the remainder of patients, about 50% will show
abnormalities when challenged by routine or cortisone glucose
tolerance tests. Another 25% of patients will have a strong family
history of diabetes. This leaves only some 10% of the total number of
patients who lack a diabetic association. 
The primary lesion of NLD is a well-defined, small, firm,
dusky-red papule topped with a fine scale. By slow enlargement or
coalescence, these lesions form indurated plaques that are round or
oblong when small and have an irregular geographic configuration when
larger. The border, which sometimes is slightly elevated, and the
adjoining skin are reddish-blue, whereas the center is yellow,
indicating lipid accumulation. The size of the lesion may vary from a
few millimeters to several centimeters. The inflammatory process
subsides, and the condition assumes its best recognized, chronic
state, that is, the sharply demarcated sclerotic plaque reminiscent
of glazed porcelain. The glossy atrophic area softens and becomes
entirely brown. Through its surfaces numerous telangiectases and
underlying larger blood vessels can be seen. 
The scale may remain fine or, particularly if ulceration is
imminent, become more prominent and collodion-like. ulceration occurs
in approximately one third of patients regardless of whether they are
diabetic. It is more common in larger lesions and may follow trauma.

Lesions of NLD are most frequent on the lower portions of the
legs, the pretibial and medial malleolar areas being the favored
sites. Lesions occasionally appear on the thighs, popliteal regions,
and feet. In 15% of cases other sites are involved in addition to the
legs. These sites include the abdomen, upper extremities(especially
the hands and forearms), and scalp, where NLD can cause atrophy and
alopecia, and the face, including the eyelids and nose. In rare cases
the condition has been noted on the heels or penis. necrobiosis
lipoidica diabeticorum also has developed in scars and at sites of
scleroderma and BCG vaccinations. Even when the lesions appear
elsewhere on the body, the legs generally are also involved.

except when they are ulcerated, the papules and plaques are
generally asymptomatic. The occasional patient will have pruritus,
burning, or tenderness. Pain, however, is a frequent companion of
ulceration. Some patients report partial or complete anesthesia of
the affected sites, suggesting local nerve dysfunction. 
As many as one in five lesions will resolve spontaneously, the
time required for improvement varies from 3 to 4 years. 
Treatment: The physician should stress the probability of
localization of lesions to the low part of the legs, the absence of
contagion, lack of malignant degeneration, and the possibility that
some areas will heal spontaneously. In patients with overt diabetes,
adequate follow-up tests for urine and blood glucose levels are
important. 
The lower parts of the legs should be protected from trauma.
Patients should be advised to avoid potentially traumatizing
situations such as certain sports and they should wear knee-length
stockings or shin pads for protection. 
In general, although many treatments have been touted, they have
little in the way of proven efficacy, and should probably be reserved
for symptomatic relief. 
Topical steroids under occlusion has been used but has not been
subjected to double-blind studies. Dermajet delivery of triamcinolone
acetonide (TMC) has shown improvement in an open study and
intralesional injection of 0.1 cc of TMC, 2.5 mg/ml, perilesionally
at 1 cm intervals has been suggested for active plaques. Clofazimine
200 mg/day cleared 6/10 patients, of 13 patients who remained on
treatment for more than one month, eight improved 
Necrobiosis lipoidica has been seen in Ehler's-Danlos Syndrome,
type VIII (OMIM
) as well as Ataxia-Telangiectasia (Thibaut
)
|
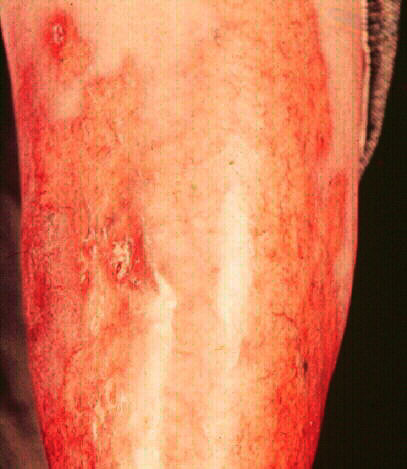
The first figure illustrates the
ulcerative form of the disease which may recrudesce
secondary to trauma. The second patient has
relatively quiescent disease with waxy tan
plaques on both shins. This lesions demonstrates the
translucency in the center portion with visibility of
underlying blood vessels. The last
photograph illustrates spontaneous resolution with residual
scarring
|

|

|
|
Necrobiosis lipoidica diabeticorum (NLD) is an uncommon
manifestation of diabetes mellitus, occurring in about 0.3% of these
patients.(55) This skin manifestation is not
pathognomonic for diabetes mellitus since less than two thirds of
patients with necrobiosis lipoidica are diabetic. Necrobiosis
lipoidica has been documented to occur prior to the onset of diabetes
mellitus.(56) Certainly any patient who presents
with necrobiosis lipoidica should be evaluated for diabetes.
The initial lesions of NLD begin as well-circumscribed
erythematous papules. Evolving radially, the sharply defined lesions
have depressed, waxy, yellow-brown, atrophic telangiectatic centers
through the underlying dermal vessels can be visualized. The
periphery is slightly raised and erythematous and there may be
partial or complete anesthesia of the lesion.(57)
Ulceration is reported in about one third of leg lesions, mostly in
large lesions following minor trauma. Lesions of NLD sometimes
spontaneously resolve, but more often they do not. They seem to occur
and persist independent of degree of control of
hyperglycemia.
Whereas most lesions of NLD occur on the legs, about 15 percent of
lesions are found elsewhere, including on the hands, forearms,
abdomen, face, or scalp. When necrobiosis lipoidica occurs in areas
other than the lower extremities, the patient is less likely to have
diabetes mellitus.(58)
The histopathology of NLD reveals neutrophilic necrotizing
vasculitis in early lesions.(59 With progression
there is collagen degeneration and destruction of adnexal structures.
Lesions evolve through granulomatous and sclerotic stages, with most
of the sclerosis occurring in the lower reticular dermis. The upper
dermis contains fatty deposits that give the lesions their yellow
color.
Electron microscopy of necrobiosis lipoidica reveals striking
changes involving dermal blood vessels consisting of focal
degeneration of the endothelial cells lining the
microvasculature.(60) These cells have electron
lucency and loss of intracellular organelles.
Treatment is used to arrest the progression of the disease. This
is most commonly achieved by application of high potency topical
steroids or intralesional injection of steroids into the active
margin. Other agents reported include pentoxifylline, high dose oral
nicotinamide(61), aspirin and
dipyridamole(62,63,64)
Currently the most impressive therapeutic option may be oral
corticosteroids. Five weeks of oral corticosteroid treatment was
described as resulting in complete disease cessation for all of six
patients treated.(65). Since the pathophysiology of
necrobiosis is not understood, it is difficult to design rational
therapy.
DIABETIC NEUROPATHY AND THE SKIN
Autonomic Neuropathy
It has been suggested that nonmyelinated nerve fibers, such as
those of the autonomic nervous system, may be the first nervous
tissue affected in diabetics.(66) In clinical
practice, evidence of autonomic neuropathy is common as manifested by
disturbance of sweating (usually anhidrosis) of the feet.
Occasionally patients complain of over-sweating elsewhere, a
compensatory mechanism for loss of the ability to temperature
regulate in the involved area. It has also been reported that
autonomic neuropathy (as measured by quantitation of the sweating
deficiency) correlates well with the severity of sensory
neuropathy.(67) One might safely assume that
patients who have diabetic sensory neuropathy also have accompanying
autonomic involvement.
The clinical manifestations of peripheral autonomic neuropathy
vary from absence of symptoms to complaints that the feet are
abnormally cold, burning, or pruritic. But there is also a problem
due to absence of sweating. Perspiration on the feet maintains
hydration of the stratum corneum: callosities without hydration tend
to become brittle and may fissure serving as a portal for infection.
Thus symptoms and signs of autonomic peripheral neuropathy in the
diabetic patient indicate the need for extra attention to foot care.

Motor Neuropathy
Diabetic motor neuropathy most often affects the foot. The
clinical presentation is wasting of the interosseous foot muscles
resulting in two major mechanical problems. The foot tends to splay
upon weight bearing, resulting in a wider foot. The toes tend to draw
upward, and the plantar fat pads move forward leaving the metatarsal
heads riding on the plantar skin without the benefit of
padding.
Motor neuropathy may appear suddenly or occur gradually over
several years. Acute and reversible motor neuropathy may follow an
episode of ketoacidosis,(68) or as the result of
insulin excess. More usual is an insidious progression of motor nerve
deterioration over many years.
Motor neuropathy in diabetes mellitus is almost always accompanied
by a sensory involvement. Changes in the shape of the foot follow the
imbalance of its internal musculature and result in ill-fitting
shoes. If the changes go unnoticed, the patient may continue to wear
shoes which can now traumatize the foot. Because of the accompanying
sensory loss, displacement of the plantar fat pads can result in
uncushioned weight bearing at the metatarsal heads. Callosities and
eventually ulceration of the weight-bearing skin or of the skin being
rubbed by the now ill fitting shoes result (neuropathic ulcers). The
presence of motor neuropathy of the foot often necessitates the use
of special widened shoes with molded inserts to redistribute weight
bearing, to accommodate and to protect the compromised foot.
Sensory Neuropathy
|

|
The erosion with callus on the tip of the toe is
typical of the type of injury which results with sensory
neuropathy of diabetes.
|

|
Mal Perforans is a particularly
devastating ulcer which is associated with underlying
osteomyelits and may presage amputation
|
Diabetics often develop sensory neuropathy on the feet, especially
with long-standing disease. The clinical presentation usually
involves tingling and numbness starting in the toes. The level of
neuropathy may vary from mild numbness of the distal toes to profound
anesthesia and neuropathic ulcers. Thermal sensitivity is also
affected. (69)
What is the clinical significance of sensory neuropathy? Although
tingling and numbness tend to be the complaint, the lack of sensation
may allow trauma to go unnoticed and result in a traumatic
ulceration. Depending upon the status of the microcirculation, these
ulcers may present difficult therapeutic problems. Neuropathic
patients who walk barefoot may sustain damage when during routine
ambulation because they have inadequate sensation to withdraw the
foot when it encounters noxious stimuli. Occasionally this unsensed
trauma during ambulation results in fracturing the bones of the feet,
eventuating into a Charcot foot.
|

|
Charcot Foot. This patient, with diabetic motor and
sensory neuropathy, developed multiple midfoot fractures
while running a short distance barefoot. The result is a
misshapen foot as seen here.
|
Patients with sensory neuropathy need to be instructed to make
sure their shoes are devoid of foreign objects before the shoes are
worn. As simple as it sounds, patients who do not follow this rule
occasionally sustain severe damage by wearing shoes which, unknown to
them, had objects (especially children's toys) included.
Other Conditions associated
with Diabetes
|

|
Bullous Pemphigoid Associated
epidemiologically with diabetes possibly secondary to
provocation by sulfonylureas.
|
|


|
Lipid Abnormalities:
Eruptive Xanthomas, (Type I Hyperlipidemia), 
Xanthelasma
|
|
 
|
Fat
Hypertrophy secondary to repeated insulin injection. Fat
atrophy may also be seen.
|
|

|
Granuloma Annulare
|
|

|
Lichen Planus
|
|
|
Diabetic Stiff-Hand Syndrome
|
SUMMARY
Diabetes Mellitus is a common ailment and virtually all persons
with diabetes develop skin manifestations of this disease. Many of
these manifestations, especially the more common ones, might be
explained on the basis of the attachment of glucose to proteins, and
the subsequent metabolism of this combination, which results in
changes in structure, function, and color. Hopefully, the common skin
findings described here may eventually be used as indicators of the
patient's current and past metabolic status.
REFERENCES
(1)Rahbar S: An abnormal hemoglobin in red cells of
diabetics. Clin Chim Acta 22:296-298, 1968.
(2)Brownlee M, Vlassara H, Kooney A, Ulrich P,
Cerami A: Aminoguanidine prevents diabetes- induced arterial wall
protein cross-linking. Science 232:1629-1632, 1986
(3)Delbridge L, Ellis CS, Robertson K, Lequesne LP:
Non-enzymatic glycosylation of keratin from the stratum corneum of
the diabetic foot. Br J Dermatol 112:547-554, 1985.
(4)Pongor S, Ulrich PC, Benesath FA, Cerami A: Aging
of proteins: Isolation and identification of a fluorescent
chromophore from the reaction of polypeptides with glucose. Proc Nat
Acad Sci USA 81:2684-8, 1984.
(5)Sell DR, Lapolla a, Odetti P, Fogarty J, and
Monnier VM: Pentosidine formation in skin correlates with severity of
complications in individuals with long-standing IDDM. Diabetes
41:1286-1292, 1992.
(6)Otsuji S, Kamada T: Biophysical changes in the
erythrocyte membrane in diabetes mellitus. Rinsho Byori 30:888-897,
1982.
(7)Greenwood AM: A study of the skin in 500
diabetics. JAMA 89:774-776, 1927
(8)Edwards JE, Tillman DB, Miller ME, Pitchon HE:
Infection and diabetes mellitus. West J Med 130:515-521,
1979.
(9)Muller SA, Winkleman RK: Necrobiosis lipoidica
diabeticorum: A clinical and pathological investigation of 171 cases.
Arch Dermatol 93:272-281, 1966.
(10)Sonck CE, Somersalo O: The yeast flora of the
anogenital region in diabetic girls. Arch Dermatol 88:846-852, 1963.

(11)Knight L, Fletcher J: Growth of Candida
albicans in saliva: Stimulation by glucose associated with
antibiotics, corticosteroids, and diabetes mellitus. J Infect Dis
123:371-377, 1971.
(12)Lugo-Somolinos A, Sanchez JL: Prevalence of
dermatophytosis in patients with diabetes: J Am Acad Dermatol
26:408-410, 1992.
(13)Tomford JW, Whittlesey D, Ellner JJ,
Tomashefski JF: Invasive primary cutaneous phycomycosis in diabetic
leg ulcers. Arch Surg 115:770-771, 1980.
(14)Petrozzi JW, Warthan TL: Malignant external
otitis. Arch Dermatol 110:258-260, 1974.
(15)Wilson DF, Pulec JL, Linthicum FH: Malignant
external otitis. Arch Otolaryngol 93:419-422, 1971.
(16)Alteras I, Saryt E: Prevalence of pathogenic
fungi in the toe-webs and toe-nails of diabetic patients.
Mycopathologia 67:157-159, 1979.
(17)Collier A, Matthews AM, Kellett HA, Clarke BF,
Hunter JA: Change in skin thickness associated with cheiroarthropathy
in insulin dependent diabetes mellitus. Br Med J 292:936,
1986.
(18)Huntley AC, Walter RM Jr: Quantitative
determination of skin thickness in diabetes mellitus: relationship to
disease parameters. Journal of Medicine, 1990, 21(5):257-64.
(10)Brik R, Berant M, Vardi P: The scleroderma-like
syndrome of insulin-dependent diabetes mellitus. Diab Metab Rev
7:121-128, 1991.
(20)Lundbaek, K: Stiff hands in long term diabetes.
Acta Med Scand 158:447-451, 1957. Rosenbloom AL, Frais JL: Diabetes
mellitus, short stature and joint stiffness - a new syndrome. Clin
Res 22:92A, 1974.
(21) Rosenbloom AL, Frais JL: Diabetes mellitus,
short stature and joint stiffness - a new syndrome. Clin Res 22:92A,
1974.
(22)Rosenbloom AL, Silverstein JH, Lezotte DC,
Richardson K, McCallum M: Limited joint mobility in childhood
diabetes mellitus indicates increased risk for microvascular disease.
N Engl J Med 305:191-198. 1981.
(23)Fitzcharles MA, Duby S, Wadell RW, Banks E,
Karsh J: Limitation of joint mobility (cheiroarthropathy) in adult
noninsulin-dependent diabetic patients. Ann Rheum Dis 43:251-257,
1984.
(24)Huntley AC: Finger pebbles: A common finding in
diabetes mellitus. J Amer Acad Dermatol 14:612-617, 1986.
(25)Clark CV, Pentland B, Ewing DJ, Clark BF:
Decreased skin wrinkling in diabetes mellitus. Diabetes Care
7:224-227, 1984.
(26)Cole GW, Headley J, Skowsky R: Scleredema
diabeticorum: A common and distinct cutaneous manifestation of
diabetes mellitus. Diabetes Care 6:189-192, 1983.
(27)Cohn BA, Wheeler CE, Briggamon RA: Scleredema
adultorum of Buschke and diabetes mellitus. Arch Dermatol 101:27-35,
1970.
(28)Konohana A, Kawakubo Y, Tajima S, Kitamura K,
Nishikawa T: Glycosaminoglycans and collagen in skin of a patient
with diabetic scleredema. Keio J Med 34:221-226, 1985.
(29)Lieberman LS, Rosenbloom AL, Riley WJ,
Silverstein JH: Reduced skin thickness with pump administration of
insulin [letter]. N Eng J Med 303:940-1, 1980.
(30)Hoerer E, Dreyfuss F, Herzberg M: Carotenemia,
skin color and diabetes mellitus. Acta Diabetol Lat 12:202-207,
1975.
(31)West KM: Epidemiology of diabetes and its
vascular lesions. New York, 1978, Elsevier North-Holland Inc., p 353.

(32)Haroon TS: Diabetes and skin--a review. Scott
Med J 19:257-267, 1974.
(33)Bernstein JE: Cutaneous manifestations of
diabetes mellitus. Curr Concepts Skin Disord 1:3, 1980.
(34)Bauer M, Levan NE: Diabetic dermangiopathy. A
spectrum including pretibial pigmented patches and necrobiosis
lipoidica diabeticorum. Br J Dermatol 83:528-535, 1970.
(35)Lithner F: Cutaneous reactions of the
extremities of diabetics to local thermal trauma. Acta Med Scand
198:319-325, 1975.
(36)Binkley GW, Giraldo B, Stoughton RB: Diabetic
dermopathy--a clinical study. Cutis 3:955-958, 1967.
(37)Fisher ER, Danowski TS: Histologic,
histochemical, and electron microscopic features of the shin spots of
diabetes mellitus. Am J Clin Path 50:547-554, 1968.
(38)Melin H: An atrophic circumscribed skin lesion
in the lower extremities of diabetics. Acta med Scand 176(Suppl
423):1-75, 1964.
(39)Danowski TX, Sabeh G, Sarver ME, Shelkrot J,
Fisher ER: Shin spots and diabetes mellitus. Am J Med Sci 251:570-5,
1966.
(40)Murphy RA: Skin lesions in diabetic patients:
The "spotted leg" syndrome. Lahey Clin Found Bull 14:10-14, 1965.

(41)Lithner F: Purpura, pigmentation and yellow
nails of the lower extremities in diabetes. Acta Med Scand
199:203-208, 1976.
(42)Ditzel J: Functional microangiopathy in
diabetes mellitus. Diabetes 17:388-397, 1968.
(43)Gitelson S, Wertheimer-Kaplinski N: Color of
the face in diabetes mellitus. Observations on a group of patients in
Jerusalem. Diabetes 14:201-208, 1965.
(44)Landau J, Davis E: The small blood-vessels of
the conjunctiva and nailbed in diabetes mellitus. Lancet 2:731-734,
1960.
(45)Grassi W, Gasparini M, Cervini C: Nailfold
computed videomicroscopy in morpho-functional assessment of diabetic
microangiopathy. Acta Diabetol Lat 22:223-228, 1985.
(46)Lithner F: Cutaneous erythema, with or without
necrosis, localized to the legs and feet--a lesion in elderly
diabetics. Acta Med Scand 196:333-342, 1974.
(47)Lithner F, Hietala S-O: Skeletal lesions of the
feet in diabetics and their relationship to cutaneous erythema with
or without necrosis of the feet. Acta Med Scand 200:155-161,
1976.
(48)Oimomi M, Maeda Y, Hata F, Nishimoto S,
Kitamura Y, Matsumoto S, Hatanaka H, Baba S: Glycosylation levels of
nail proteins in diabetic patients with retinopathy and neuropathy.
Kobe J Med Sci 31:183-188, 1985.
(49)Rocca F, Pereyra E: Phlyctenar lesions in the
feet of diabetic patients. Diabetes 12:220-222, 1963.
(50)Allen GE, Hadden DR: Bullous lesions of the
skin in diabetes (bullous diabeticorum). Br J Dermatol 82:216-220,
1970.
(51)Cantwell AR, Martz W: Idiopathic bullae in
diabetics. Bullosis diabeticorum. Arch Dermatol 96:42-44,
1967.
(52)Kurwa A, Roberts P, Whitehead R: Concurrence of
bullous and atrophic skin lesions in diabetes mellitus. Arch Dermatol
103:670-675, 1971.
(53)James WD, Odom RB, Goette DK: Bullous eruption
of diabetes mellitus. A case with positive immunofluorescence
microscopy findings. Arch Dermatol 116:1191-1192, 1980.
(54)Bernstein JE, Medinica M, Soltani K, Griem SF:
Bullous eruption of diabetes mellitus. Arch Dermatol 115:324-325,
1979.
(55)Muller SA: Dermatologic disorders associated
with diabetes mellitus. Mayo Clin Proc 41:689-703, 1966.
(56)Ellenberg M: Diabetic complications without
manifest diabetes. JAMA 183:926-930, 1963.
(57)Boulton AJ, Cutfield MB, Abouganem D, Angus E,
Flynn HW, Skyler JS, Penneys NS: Necrobiosis lipoidica diabeticorum:
a clinicopathologic study. J Am Acad Dermatol 18:530537,
1988.
(58)Wilson Jones E: Necrobiosis lipoidica
presenting on the face and scalp. Trans St Johns Hosp Dermatol Soc
57:202, 1971.
(59)Ackerman AB: Histologic diagnosis of
inflammatory skin diseases. A method by pattern analysis.
Philadelphia, 1978, Lea & Febiger, pp. 424-431.
(60)Heng MCY, Allen SG, Song MK, Heng MK: Focal
endothelial cell degeneration and proliferative endarteritis in
trauma-induced early lesions of necrobiosis lipoidica diabeticorum.
Am J Dermatopath 13:108-114, 1991.
(61)Handfield-Jones S, Jones S, Peachey R: High
dose nicotinamide in the treatment of necrobiosis lipoidica. Br J
Dermatology 118:693-696, 1988.
(62)Eldor S, Diaz EG, Naparstek E: Treatment of
diabetic necrobiosis with aspirin and dipyridamole. N Engl J Med
298:1033, 1978.
(63)Fjellner B: Treatment of diabetic necrobiosis
with aspirin and dipyridamole. N Engl J Med 299:1366, 1978.
(64)Unge G, Tornling G: treatment of diabetic
necrobiosis with dipyridamole. N Engl J Med 299:1366, 1978.
(65)Petzelbauer P, Wolff K, Tappeiner G:
Necrobiosis lipoidica: treatment with systemic corticosteroids. Br J
Dermatology 126:542-545, 1992.
(66)Martin MM: Involvement of autonomic nerve
fibers in diabetic neuropathy. Lancet 264:560-565, 1953.
(67)Kennedy WR, Sakuta M, Sutherland D, Goetz FC:
Quantitation of the sweating deficiency in diabetes mellitus. Ann
Neurol 15:482-488, 1984.
(68)Brown MJ, Asbury AK: Diabetic neuropathy. Ann
Neurol 15:2-12,1984.
(69)Navarro X, Kennedy WR: Evaluation of thermal
and pain sensitivity in type I diabetic patients. J Neurol Neurosurg
Pshchiatry 54:60-64, 1991.
(70) Robert JJ, TI - Hyperinsulinism syndromes
caused by insulin resistance TT - Exploration des syndromes
d'hyperinsulinisme par resistance a l'insuline. 
(71) Thibaut, S.; Sass, U.; Khoury, A. and Simonart, J.-M.:
Ataxia-telangiectasia and necrobiosis lipoidica: an explainable
association. Europ. J. Derm. 4: 509-513, 1994. 
© 1995-2000 The Internet Dermatology Society, Inc. All rights
reserved.
Send your comments to: textbook@telemedicine.org






































